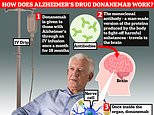
Patient groups reacted with anger today as health chiefs blocked NHS patients from getting a breakthrough Alzheimer’s drug proven to tackle the devastating disease.Donanemab has been hailed as ‘a new hope’ in the fight against dementia, after studies showed it slowed the memory-robbing illness in its early stages.And today it got the green light from UK medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA). But in a blow Britain’s near-one million dementia patients and their families, the NHS spending watchdog ruled the benefits were ‘not enough’ to justify the cost. This means donanemab will only be available to those who can afford to stump up £25,000 a year for the drug alone. Experts have long believed donanemab could herald a new era of dementia treatment, after studies showed it slowed the memory-robbing illness in its early stages Alzheimer’s disease is the most common cause of dementia. The disease can cause anxiety, confusion and short-term memory lossIt is also believed health insurance policies are unlikely to cover costs.Campaigners and charities today criticised the decision, labelling it ‘incredibly disappointing’ and ‘a frustrating setback’.It is estimated around 70,000 adults in England would have been eligible for donanemab, if approved for NHS use.It is also the second time a new Alzheimer’s treatment has been rejected by watchdog the National Institute for Health and Care Excellence (NICE) in a matter of months. In August, it ruled that the benefits of lecanemab, which was also approved by the MHRA, as ‘too small’ to justify the cost.Hilary Evans-Newton, chief executive at Alzheimer’s Research UK, said: ‘Today’s announcement marks another frustrating setback for people affected by Alzheimer’s disease. ‘We finally have two new treatments licensed in Britain for Alzheimer’s.’But it’s incredibly disappointing that NHS patients in England and Wales won’t receive them. ‘While these drugs are not cures and come with risk of side effects, trials show they are the first treatments to slow the decline in memory and thinking skills linked to Alzheimer’s, rather than just alleviating symptoms.’Dementia remains the UK’s leading cause of death, and without action, an ageing population means more families will be affected, driving up NHS costs through emergency admissions and care.’Donanemab has been proven to slow progression of the disease by as much as 35 per cent in trials by helping to remove the build up of the harmful protein amyloid in the brains of people with early-stage Alzheimer’s. Known as an amyloid immunotherapy, it is thought that these proteins interfere with messages sent between different parts of the brain, resulting in the memory and independence robbing symptoms. Patients received donanemab through a drip in the arm in hospital, every months for 18 months in clinical trials. But experts said additional monitoring for side effects including brain scans mean additional costs could spiral. The drug has been proven to slow progression of the disease by as much as 35 per cent in trials by helping to remove the build up of the harmful protein amyloid in the brains of people with early-stage Alzheimer’s Around 900,000 Brits are currently thought to have the memory-robbing disorder. But University College London scientists estimate this will rise to 1.7million within two decades as people live longer. It marks a 40 per cent uptick on the previous forecast in 2017Explaining its decision, Helen Knight, director of medicines evaluation at NICE said: ‘For NICE to be able to approve a medicine for use in the NHS it must provide additional benefits to patients, and it must also represent a good use of NHS resources and taxpayers’ money.’Our independent committee looked at all the available evidence. This shows donanemab could slow down cognitive decline by four to seven months, but this is just not enough benefit to justify the additional cost to the NHS.’The cost-effectiveness estimate for donanemab is 5 to 6 times above what NICE normally considers an acceptable use of NHS resources.’I know this will be disappointing news, but this is an emerging field of medicine and there are other treatments being developed.’NICE also said it had identified 27 other drugs which it ‘expects to be asked to evaluate over the next few years’. NICE also said it had identified 27 other drugs which it ‘expects to be asked to evaluate over the next few years’Calling on the Secretary of State for Health and Social Care, Wes Streeting, to step up Ms Evans-Newton, added: ‘NHS England has identified nearly 30 other dementia treatments that could be available by 2030, giving the Government and NHS a crucial opportunity to transform how dementia is treated — just as Labour pledged in their manifesto. ‘But we still haven’t heard from Health Secretary Wes Streeting on how he plans to break the deadlock we’re facing, where research is delivering new treatments but they remain out of reach for NHS patients. Professor Fiona Carragher, Chief Policy and Research Officer at Alzheimer’s Society, added: ‘Disease-modifying therapies like donanemab and lecanemab offer a new horizon of hope in the fight against dementia.’We need to see significant government investment to bring about radical change so that everyone with dementia in the UK can get an early and accurate diagnosis. ‘Without this, people won’t be able to access existing treatments and interventions to help manage their symptoms today or be ready for the disease slowing treatments of tomorrow.’Rob Howard, Professor of Old Age Psychiatry, University College London said he believed NICE had made ‘the right decision’.He said that the ‘small benefits’ of donanemab may not last longer than 18 months, and that ‘NHS access to these new drugs would not have made an appreciable difference to the experience of patients and families affected by dementia’.Prof Howard added: We have well-established drug treatments and psychosocial interventions for Alzheimer’s disease that are already available to people with dementia within the NHS but are not universally accessed. ‘Our priority now should be to ensure that everyone with dementia who might benefit from these cost-effective interventions and adequately resourced adult social care services is able to access them. ‘It would be unhelpful if the conversation about how we adequately fund NHS and social care for people with dementia was distracted by the issue of these new drugs.’ Recent analysis by the Alzheimer’s Society estimates the overall annual cost of the dementia to the UK is £42billion a year, with families bearing the brunt.
Your browser does not support iframes.
An ageing population means these costs – which include lost earnings of unpaid carers – are set to soar to £90billion in the next 15 years.Around 944,000 in the UK are thought to be living with dementia, while the figure is thought to be around 7million in the US.Alzheimer’s affects around six in 10 people with dementia.It is thought to be caused by a build-up of amyloid and tau in the brain, which clump together and from plaques and tangles that make it harder for the brain to work properly.Eventually, the brain struggles to cope with this damage and dementia symptoms develop.Memory problems, thinking and reasoning difficulties and language problems are common early symptoms of the condition, which then worsen over time.Dementia are expected to sky-rocket in the coming years, making a cheap screening tool vital to get to grips with the challenge.Alzheimer’s Research UK analysis found 74,261 people died from dementia in 2022 compared with 69,178 a year earlier, making it the country’s biggest killer.